| Diffusion Coefficient of Gas, Liquid & Vapour in Polymer |
If you want to simulate diffusion, permeation and chemical resistance of polymer based materials, we invite you to check our new tool: CheFEM prologue.
The diffusion coefficient or so called diffusivity has the dimensions of [length2 time-1], [m2 s-1]. These dimensions result from the underlying kinetic theory. The diffusion theory states that chemicals move with a certain molecular velocity, ![]() [m/s], depending on particle size and temperature, along a free path,
[m/s], depending on particle size and temperature, along a free path, ![]() [m]. The free path length is determined by the amount of matter per cubic meter. The less matter is available, the longer the available path length. Hence, self diffusion rates of gases are much higher than liquids.
[m]. The free path length is determined by the amount of matter per cubic meter. The less matter is available, the longer the available path length. Hence, self diffusion rates of gases are much higher than liquids.
Then, it is hypothesized that the chance of travelling one times the free path distance in the positive x-direction is equal to 1; the chance of travelling two times the free path in the positive x-direction is 1/2; the chance of travelling four times the free path in the positive x-direction is 1/4, and so on. One can imagine that this yields the following expression for the diffusivity:
![]() (1)
(1)
Since we live in a three dimensional world, we have to add the chance of going into the positive x-direction (we could also have gone into the - x, +y, -y, +z, -z direction):
![]() (2)
(2)
Watch species diffusie in real life (including a calculation of the diffusion coefficient): the video on Brownian movement.
Diffusion Distance in Fick's Laws
Fick's First Law adds a driving force - the concentration gradient - to the diffusion coefficient. This enables one to calculate the diffusion flux or mass transport in a preferential direction of for example water, a solvent or natural gas into a coating, packaging polymer, multilayer plastic , fibre metal laminate or glass fibre composite. Moreover, by solving the partial differential equation of Fick's Second Law we can define a function c(x,t) that gives the concentration of the diffusing species as a function of time and place in unsteady conditions. As long as the medium is semi-infinite (penetration from one side), the diffusion coefficient of species in polymer, multilayer and composite materials can be calculated from the weighted average distance travelled. This distance is calculated from the concentration function as follows:
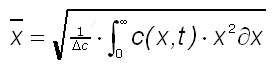 (3)
(3)
With delta c the concentration gradient. The diffusion coefficient now follows from:
 (4)
(4)
If Fick's First Law applies, the penetration depth for small times (Fourier Mass number << 0.1) follows from:
![]() (5)
(5)
Determination of Diffusion Coefficients
Often the so called time lag method is used to determine the diffusion coefficient of molecules through plastic materials. In this method the polymer or composite sample is exposed on one side to the gas, liquid, solvent or vapour of interest. On the opposite side, the concentration of molecules is continuously measured by use of analytical equipment. At the same time, species are removed on this side to prevent concentration build-up. Then, after a certain time a steady state diffusion flux is obtained. This time relates to a weighted average diffusion distance. If the diffusion coefficient is constant, this steady state distance is calculated as follows:
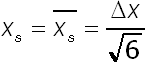 (6)
(6)
With delta x is the thickness of the polymer based material. The related time lag formula is then:
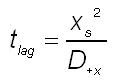 (7)
(7)
From the experiment, the time and distance is known. Hence, the diffusion coefficient can be calculated. The reader is warned that this formula for time lag can applies when (i) diffusion is governed by Fick's First and Second Law (no pressure gradients or other driving forces than concentration gradients involved), (ii) when the diffusion coefficient is constant and not a function of concentration (when the polymer or composite material swells) or distance (such as the case in multilayer and fibre reinforced composite materials)!
Case Stories
Using Epoxy Coating for Pipeline Retrofitting
In the near future, natural gas pipelines (type X-52, X-60, X-65 and X-70) may also
be used for transport of Hydrogen or even Carbon Dioxide. In case of Hydrogen
conveyance, Hydrogen might be mixed with Natural Gas (parallel gas transport,
using a membrane to seperate the gases at the outlet) or transported solely.
Discarded natural gas pipelines may also be completely retrofitted for Hydrogen or
Carbon Dioxide transport. With regard to Hydrogen, a service life concern could be
HISC (Hydrogen Initiated Stress Cracking) of steel. Hydrogen embrittlement or HISC
results from combining of diffusing Hydrogen atoms into molecular Hydrogen - or
the formation of molecular Methane - in internal metal voids of nanoscopic size.
The generated pressure - in combination with intrinsic circumferential stress in the
material - can exceed the restrain pressure of steel, especially near the loading
surface. Whether HISC is an issue is largely dependent on the sort of steel,
internal pressure and temperature.
Read using Epoxy Coating for Pipeline Retrofitting
Carbon Dioxide Diffusion Loss from PET Bottle
An interesting case is the diffusion of CO2 (carbon dioxide) through the wall of a
PET
(Polyethylene Terephthalate) bottle that contains soft or alcoholic drinks. You
can think of
cola or beer. Reason for putting gaseous CO2 in drinks is taste.
The gas gives a sparkling
feeling and a reduction in tasted sweetness.
One can imagine that diffusion of CO2
through the wall is a problem because of loss
of these features in time; in other words, a
loss of quality in time. In this case story,
the CO2 diffusion from a bottle as a function of time is demonstrated in real-life
circumstances.
Read Carbon Dioxide Diffusion from PET
Composite Water Permeation and Chemical Resistance
In new composite equipment, such as in aerospace parts, body
parts for car and trains, electronic packages, medical packaging, membranes for
simultaneous carbon dioxide / water vapour removal and other membrane
applications, the mechanical retention if the composite is exposed to hygrothermal
conditions is one of the main interests.
The fact that water diffuses with a considerable rate in polymers, in combination with
the anomalous diffusion and uptake behaviour, have lead to a variety of different
theories with regard to water behaviour in composites. In this preliminary paper we
present our view on water diffusion and mechanical retention in an unreinforced and
glass fibre reinforced Derakane 411 Epoxy Vinyl Ester Resin.
We have used CheFEM to interpret gravimetric data using two main concepts:
[1] localized water adsorption theory, [2] multilayer diffusion simulation.
The composite configuration that we tested did not show chemical degradation, but
swells to a small extent under the continuous load of water.
Read composite chemical resistance in hygrothermal conditions
Polyimide for Flexible Thin Film Solar Cells
One of the candidate substrate materials for polymer based solar cells and Organic
Light Emitting Diodes (OLED's) are transparant polyimides. Because of their
combination of mechanical, moisture barrier and corrosion resistance (among which
are UV light and extreme high Oxidation resistance) properties - even at high
temperatures - they might be suitable.
In this case study the water absorption characteristics of a new polyimide in real life
circumstances is evaluated. By means of gravimetric, free volume and electrical
impedance (EIS) data, it is found that water absorption in this specific polyimide can
be rigorously modelled using the dual mode sorption model. According to this
model, a part of the water molecules is adsorbed on hydrophilic sites in the polymer
with a Langmuir isotherm. This part is immobile. Another part dissolves in the
polymer and can move by normal (Fickian) diffusion. The implications of this
significant anomaly on water breakthrough times, on water mass flux into the
device, on accelerated weathering tests and on dimensional change are explained
thoroughly.
Read water diffusion polyimide based solar cells
Lifetime Prediction of Thermoplastic Joint for Bridge Decks
Thermoplastics joints have been developed to solve the corrosion problem of
bridges, sluices, dams and other structures. The different steel reinforements are to
a greater or lesser extent susceptible to corrosion in the highly alkaline environment
of concrete. As a consequence, the thermoplastic joint could provide some basic
protection for the less durable materials solutions against diffusion and
deterioration. In this paper, the combined aqueous immersion and sustained stress
on the service life of a plastic joint is chararacterized. This is done by mechanical
testing and simultaneous chemicals exposure.
Read osmosis in composite materials
Read GFRP Rods in Concrete
100% Nitrogen in Car/Motor Cycle Tyres
Usually we fill our tyres with Air. Nowadays there are several discussions in
magazines and internet on whether it would be better to fill tyres with 100%
Nitrogen or Carbon Dioxide instead of Air. The diffusion resistance layer of tyres are
made from a polymer rubber called Polyisobutylene, and a few percent of Isoprene.
In this case history it is shown that this polymer has a better diffusion resistance
against Nitrogen than Oxygen. Moreover the practical implications of this difference
are described.
Read pure nitrogen in tyres
Methanol diffusion in GFRP
Here we demonstrate how mass transfer of liquids and saturated vapours is applied
in an industrial application. We were commissioned to assess whether it is possible
to use a GFRP (Unsaturated Polyester Glass Fibre Reinforced Plastic) pipeline for the
transport of methanol. Apart from past effects of alkaline water transport on
sustainability of this pipeline, it is shown how the methanol flux changes in the time
period evaluated. In this example the counter diffusion of polluting chemicals in
ground water was not taken into account.
Read Methanol Diffusion GFRP
Polyethylene Liner on Tank for Gasoline
In a wide range of industries, various composite and lining systems are used with
the objective to combine good mechanical and chemical resistance properties. Key to
a sustainable and - sometimes even - a multi-purpose service life, is chemical
resistance and diffusion analysis in an early development stage. The case of gasoline
in a HDPE lined / coated Epoxy tank gives a clear insight in our approach. We have
more information available on similar configurations, like hoses, tubes, adhesives,
rubbers and elastomers for gasoline based on PA, PVDF, PPS including different
kinds and degrees of reinforcements.
Read Gasoline Diffusion HDPE Epoxy
Using Hildebrand Solubility Parameters for Material Selection
We want to assess what sort of thermoplastic polymer we can use for containment
of toluene at ambient conditions. A special design requirement is prevention of
plasticizing of the material by toluene. This requirement is related to diffusion
resistance: in many instances plasticizing causes the initial diffusion rate to increase
several orders of magnitudes. This can be noticed from the figures in the table for
liquid diffusion on the website. If a chemical has a high solubility in the polymer, then
the weighted average diffusion rate (D|) becomes much larger than the initial rate
(D0).
Read solubility parameters for polymer material decision
Hygrothermal Resistance of Epoxy - Polyester Composites
In glass fibre reinforced plastics (GFRP) or glass fibre metal laminates (FML's) the
combination of the different components do substantially increase the mechanical
properties of the material, think for example of high toughness, very long critical
crack lengths, etc. At the same time the behaviour of these materials when exposed
to chemicals, high pressure and high temperature is becoming more and more
complex.
For fully integrated chemical-physical service life time predictions, the situation at the
interface should often be the focal point of analysis and simulation. Here, possible
voids do appears, temperature or swelling stress beyond the interfacial strength
may come into play, cohesive strength, adhesive work, interlaminar shear strength
values, pull and peel tests, etc. The attached paper is one of our first works on this
subject and was published in the framework of ESAT 2006.
Read Osmosis in composite materials
Service Life Behaviour of Thin Film Flexible Solar Cells
In this case study the lifetime of a thin film flexible solar cell is assessed.
The multilayer solar cell will be applied in curved body parts for vehicles.
The long term mechanical and corrosion resistant properties are focus of the study.
For sake of corrosion prevention of the subsequent functional layers, on one hand
crystallinity and thickness of the transparent surface polymer must be as high
as possible. As such, the permeation rate of Oxygen and Water through the surface
layer is kept low. On the other hand, adhesion of the polymer to the substrate
decreases as function of thickness. The robustness of the interface is of
major importance in restraining the mass solubility and temperature driven
expansion stresses and mechanical stresses at the surface. To complicate things
even more, the impact resistance / fracture toughness of the surface polymer
decreases as function of the degree of crystallinity.
Read lifetime of a thin film flexible solar cell
References
[1] Einstein, A., Investigations on the theory of the Brownian movement, Dover Publ. (1956)
[2] Frisch, H.L., Time lag in transport theory, Journal of Chemical Physics, 36, 2(1962)
[3] Cranck. J, The Mathematics of Diffusion, Oxford Clarendon Press (1956)
[4] Cranck, J.; Park G.S., Diffusion in Polymers, Academic Press London
[5] Dlubek, G.; et al., Free Volume Variation in Polyethylenes of Different Crystallinities: Positron Lifetime, Density and X-Ray Studies, J. of Pol. Sci., Part B, 40, 65-81 (2001)
[6] Wesselingh J.A.; Krishna R., Mass Transfer in Multicomponent Mixtures, Delft University Press (2000)
Internal Links
-More on temperature dependence of diffusion, solubility & permeability.
There are number of analytical- experimental techniques which can be used to determine the diffusion coefficient through polymer interfaces. They are summarized as follows:
1. Scanning infrared microscopy
- infrared microdensitometry
- scanning infrared microscopy
2. light scattering
- optical Schlieren technique
- spectroscopic ellipsometry
- dynamic light scattering
3. Neutron scattering
- small-angle neutron scattering (SANS)
- neutron reflection spectroscopy (NRS)
4.Raman scattering
-surface-enhanced Raman scattering (SERS)
5. Infrared spectroscopy
- external reflection infrared spectroscopy
- attenuated total reflectance spectroscopy
- transmission FTIR
- reflection absorption spectroscopy
- attenuated total reflection microspectrometry.
6 Other methods
- Photon correlation spectroscopy
- Donor-acceptor fuorescence method
- Small-angle x-ray scattering (SAXS)
- Electron microprobe analysis
- Nuclear reaction analysis (NRA)
- Ellipsometry
- Electrical (Electro Chemical) Impedance Spectroscopy (EIS)
- Electrochemical Noise Spectroscopy / Measurement (ENM)
1. Are these electrochemical methods also the preferred methods for measuring diffusion coefficients of a polymer or polymer based material on a substrate?
2. Or are they only suitable for measuring a general degradation state of the coating or polymer without detailed information on primarly diffusion rate, thermodynamic information, chemical corrosion rates and interfacial issues?
3. Does anyone have experience with a continuous Electrical Impedance Spectroscopy (EIS) coating measurement in the field (oil platforms, vessels, bridge, etc.) and the forthcoming results regarding the diffusion coefficient?
Thanks Ralph
p.s I am mainly interested in polyurethane / epoxy based coatings on metals (Zinc, Aluminum, Stainless Steel) and exposure to weathering conditions